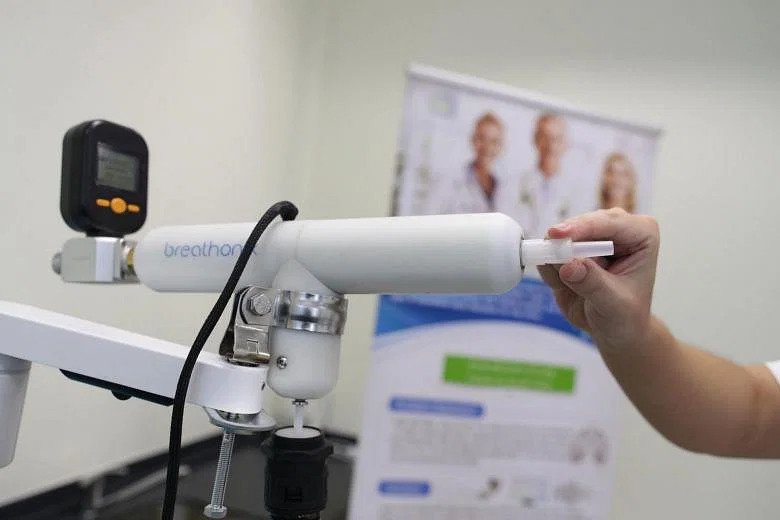
SINGAPORE – More people here may soon be able to get tested for Covid-19 and know their results in less than a minute, simply by breathing into a tube.
A test system that can accomplish this has been developed by National University of Singapore (NUS) spin-off Breathonix.
Following a successful trial at the National Centre for Infectious Diseases (NCID), the company said on Thursday (Oct 29) that it is currently in discussions with the Health Ministry (MOH) to deploy its breathalyser tests in trials at public locations in the coming months.
Dr. Jia Zhunan, chief executive of Breathonix, said the firm is also working to expand the NCID trial to include a further 250 patients in addition to the current 180.
The company’s chairman, Associate Professor Neo Kok Beng, added that they are in talks with a “major hospitality player” here to deploy the tests at such events as conferences following approval from the Health Sciences Authority (HSA).
The company hopes to receive it in the first quarter of 2021.
Prof Neo declined to name the hospitality player. He added that Breathonix has also received some requests from groups overseas for the tests to be rolled out there.
For the test, the user exhales into a disposable one-way valve mouthpiece that is connected to the breath sampler.
A mass spectrometer analyses the invisible particles called volatile organic compounds (VOCs) in a person’s breath.
A healthy person will have a different VOC signature from someone who is ill, and different illnesses produce different signatures.
The results are generated automatically within a minute, without the need for the sample to be processed elsewhere.
This makes the breathalyser test more convenient and faster than the current polymerase chain reaction (PCR) swab tests, which require an external laboratory to process samples, and take a few days for results to be returned.
The results of Breathonix’s test are also generated faster than those of antigen rapid tests (ART), which take at least 15 minutes.
The breathalyser test is also non-invasive, in contrast to the PCR tests and ARTs which require swabs to be inserted into a person’s nostrils and have been known to cause discomfort.
Additionally, unlike a PCR test which requires skilled laboratory technicians to process samples, Breathonix’s machine requires only about an hour of training for a layman to operate, said the company’s chief operating officer and co-founder Mr Du Fang.
“Technically, anyone who knows how to operate a PC can do it,” he added.
At the recent NCID clinical trial, the breathalyser test also managed to pick up asymptomatic patients – although Dr. Jia cautioned that further studies and trials are needed to validate the results.
It has a 93 per cent sensitivity rate – the rate at which positive cases are correctly picked up – and a 95 per cent specificity rate, which is the proportion of people who are virus-free that are correctly identified as such.
In contrast, the ARTs used at a recent pre-event testing pilot here had a sensitivity rate of about 82 per cent and a specificity rate of 99 per cent.
The breathalyser is less sensitive than a PCR test, which is considered the “gold standard” of Covid-19 detection here, but Dr. Jia pointed out that the breath test is not meant to be a diagnostic one.
“We’re not comparing or competing against the PCR test. The breath test is more of a first-level screening device,” she explained. For instance, if the breath test shows negative, then the test subject is cleared. If the breath test shows positive, then the test subject is recommended to undergo the PCR test as a confirmation.
The exact cost of a breathalyser test here will depend on several factors, including the number of tests conducted, but Prof Neo said that if around 5,000 people are tested per month using one machine, each test should cost around US$20 (S$27).
Currently, a PCR test here is estimated to cost around $200 per person. Mr. Du added that as ARTs are currently still under validation here, it is not known how much such tests cost.
Dr. Jia said that there are several measures in place to prevent cross-contamination when using the machine. These include a disposable mouthpiece and disposable tubing, a one-way valve, and a filter on top of the mouthpiece so only gas-based molecules can enter the device.
In addition, all surfaces inside the machine in contact with the breath are heated to a high temperature and are self-sanitizing.
Prof Neo added that the machine is able to run non-stop, 24 hours a day, for up to six months before it needs to be re-calibrated. This, combined with the quick and painless testing method, makes it ideal for deployment at high-traffic flow places such as airports.
He said: “If you ask people to stay another two days or even 10 hours at the airport (while waiting for results), that is not going to be encouraging… so we have to look at the bigger picture, rather than saying this is just a test: How does it facilitate the reopening of the economy?”
He added: “We believe we can contribute to the global economy through fast, accurate and cost-effective screening.”
Source: https://www.straitstimes.com/singapore/health/covid-19-breathalyser-tests-may-be-deployed-more-widely-in-trials-here-before-q1
